Michaela Th. Mayrhofer is a political scientist and historian by training. She was educated in Vienna, Louvain-la-Neuve, Essex and Paris. In 2010, she has earned her PhD from both the Ecole des Hautes Etudes en Sciences Sociales and the University of Vienna, which was shortlisted by the Austrian Society for Political Science for 'best thesis 2010'. Prior to her involvement in BBMRI-ERIC, she was investigator in several national and international research projects focusing on the politics of biotechnology and the life sciences, especially the governance of biobanks. Her academic career led to various positions at the Centre de Recherche Médecine, Sciences, Santé et Société, the University of Vienna, the Institute of Science, Technology and Society Studies at Alpen-Adria-Universität Klagenfurt/ Vienna/Graz, the Technical University of Vienna and the Medical University of Graz. Today, she serves as the Chief Policy and Coordination Officer of BBMRI-ERIC and coordinates the Code of Conduct for Health Research initiative.
About CINECA:
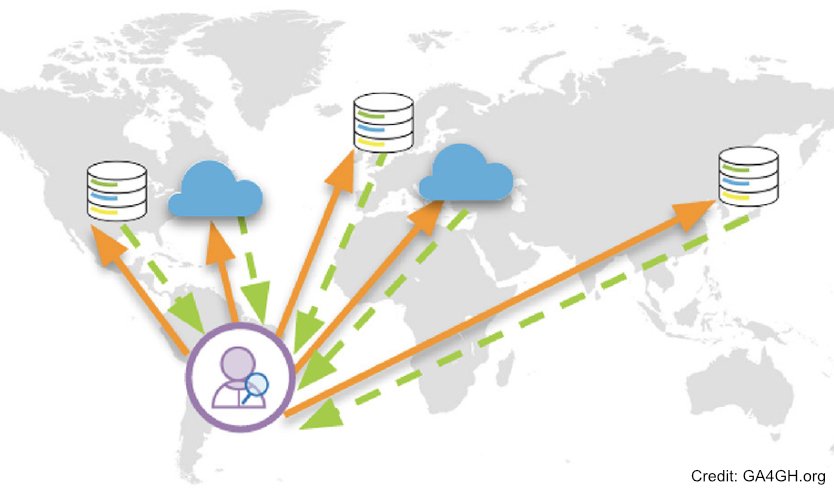
The CINECA (Common Infrastructure for National Cohorts in Europe, Canada, and Africa) project aims to develop a federated cloud enabled infrastructure to make population scale genomic and biomolecular data accessible across international borders, to accelerate research, and improve the health of individuals across continents. CINECA will leverage international investment in human cohort studies from Europe, Canada, and Africa to deliver a paradigm shift of federated research and clinical applications. The CINECA consortium will create one of the largest cross-continental implementations of human genetic and phenotypic data federation and interoperability with a focus on common (complex) disease, one of the world’s most significant health burdens. CINECA has assembled a virtual cohort of 1.4M individuals from population, longitudinal and disease studies. Federated analyses will deliver new scientific knowledge, harmonisation strategies and the necessary ELSI framework supporting data exchange across legal jurisdictions enabling federated analyses in the cloud. CINECA will provide a template to achieve virtual longitudinal and disease specific cohorts of millions of samples, to advance benefits to patients. It will leverage partner membership of standards and infrastructures like the Global Alliance for Global Health, BBMRI, ELIXIR, and EOSC driving the state of the art in standards development, technical implementation and FAIR data.